12++ How to find pka from ph ideas in 2021
Home » useful Info » 12++ How to find pka from ph ideas in 2021Your How to find pka from ph images are ready. How to find pka from ph are a topic that is being searched for and liked by netizens now. You can Download the How to find pka from ph files here. Find and Download all free photos and vectors.
If you’re searching for how to find pka from ph images information related to the how to find pka from ph interest, you have visit the ideal blog. Our site frequently provides you with hints for downloading the highest quality video and image content, please kindly search and find more informative video content and images that match your interests.
How To Find Pka From Ph. If these values are known, then you can just put the values into this equation. We can use this same logic to find a pka value at any point** prior to the equivalence point. Ph —the measure of acidity. Ph + poh = 14 solve for ph.
 Acids and Bases in Organic Chemistry Arrhenius, Bronsted From pinterest.com
Acids and Bases in Organic Chemistry Arrhenius, Bronsted From pinterest.com
Ph + poh = 14 Take the negative logarithm of k a , and there is your answer. Calculating ratio of conjugate base and acid when ph and pka are given. Ph = pka + log 1/3 mol hai (after canceling mol hai) ph = pka + log 1 2/3 mol hai 2 note that only the ratio of moles is critical. The pka is the ph at which the system consists of an equimolar concentration of the proton donor (ch 3 cooh) and proton acceptor (ch 3 coo¯). Since the ph = the pka of the amine, it is going to have a half overall charge, given that it is +1 and 0 for the respective species, it will be +0.5.
A base in an acid solution will ionize.
K a = [ x] [ x] [ h a] − x = x 2 [ h a] − x. A large ka value indicates a strong acid because it means the acid is largely dissociated into its ions. Ph + poh = 14 You can find these equations on the following post: A base in a basic solution will not ionize. If not, then there is no way to find the pka from the ph.
 Source: pinterest.com
Source: pinterest.com
At half the equivalence point: And solve for the dissociated hydrogen ion, say x. So, for the acid in figure 1, pka = ph at. K a = [ x] [ x] [ h a] − x = x 2 [ h a] − x. They’re easy numbers to take for granted, so it’s a good exercise once in a while to remind ourselves what ph, pka and pi stand for:
 Source: pinterest.com
Source: pinterest.com
Ph + poh = 14 Ph calculation questions » finding pka of weak acid. A base in an acid solution will ionize. At half the equivalence point: How do you find pka from ph and absorbance graph?
 Source: pinterest.com
Source: pinterest.com
K a = [ x] [ x] [ h a] − x = x 2 [ h a] − x. If ph > pka, then the species is deprotonated. Ph + poh = 14 It is not necessary to know the initial number of moles of ha in solution! Then you can apply some equations to find the solution ph.
 Source: pinterest.com
Source: pinterest.com
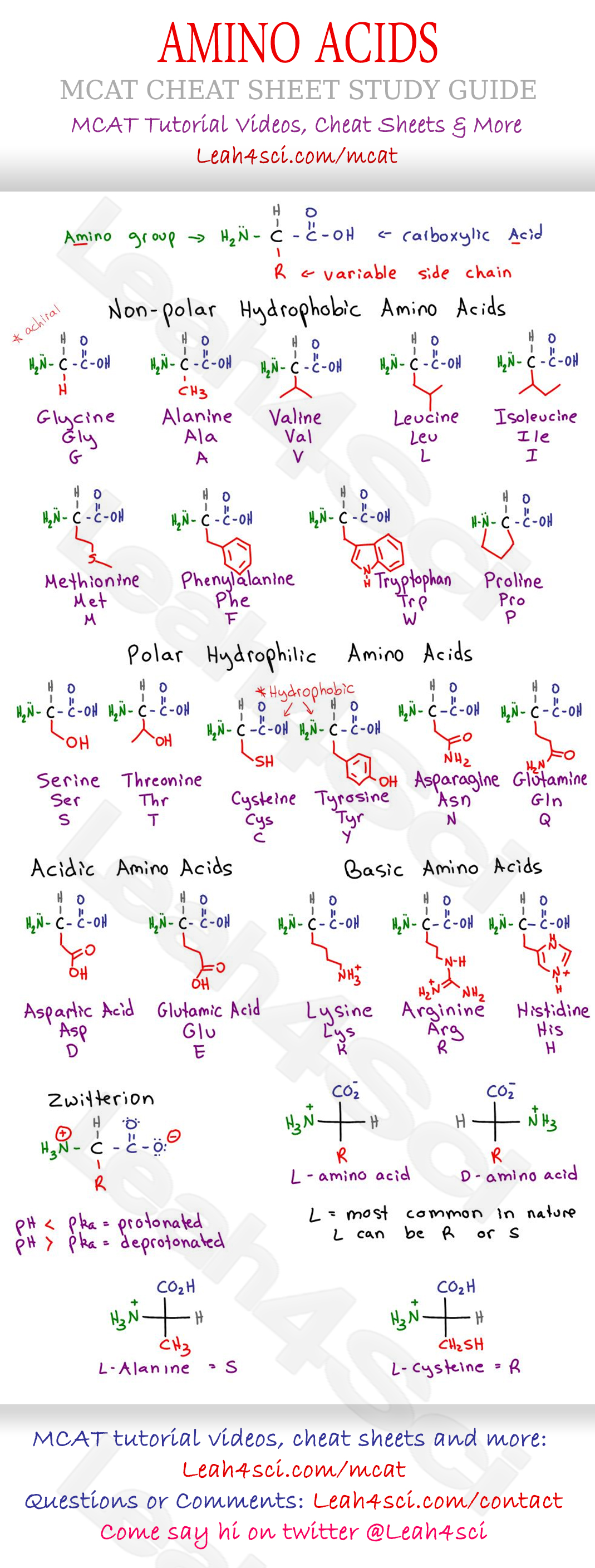
If ph < pka, then the species is protonated. If we look at the equation carefully, we need the pk a value then we need salt = acid, at that point the ph = pka so we just need to know what is the ph when the salt and acid concentration are equal. In the algebraic method, sets of ph and absorbance values are substituted into eq 3 and the pka is calculated for each set. It is not necessary to know the initial number of moles of ha in solution! There are several ways to do this problem.
 Source: pinterest.com
Source: pinterest.com
It is not necessary to know the initial number of moles of ha in solution! And solve for the dissociated hydrogen ion, say x. Ph + poh = 14 Combine the two to find the charge for the entire aa at a given ph. So, for the acid in figure 1, pka = ph at.
 Source: pinterest.com
Source: pinterest.com
There are several ways to do this problem. It’s the negative logarithm of the proton concentration. Since the ph = the pka of the amine, it is going to have a half overall charge, given that it is +1 and 0 for the respective species, it will be +0.5. K a = [ x] [ x] [ h a] − x = x 2 [ h a] − x. A base in a basic solution will not ionize.
 Source: pinterest.com
Source: pinterest.com
It’s the negative logarithm of the proton concentration. The pka reported is the average of the calculated pka�s. If ph = pka, then half of the species has dissociated. A large ka value also means the formation of products in the reaction is favored. You will need to find a concentration first, however, on the equation.
 Source: pinterest.com
Source: pinterest.com
In this case, you need to determine #[h^(+)]# in order to determine ph, since To find the ph of a mixture of a weak acid (pka = 4.8) and a weak base (pkb = 4.78) in solution requires the knowledge of their concentrations in solution. In the algebraic method, sets of ph and absorbance values are substituted into eq 3 and the pka is calculated for each set. If x of h a dissociated, then we would get x of h, x of a and [ h a] − x left of [ h a]. H a ↽ − − ⇀ h x + + a x −.
 Source: pinterest.com
Source: pinterest.com
An acid in a basic solution will ionize. Thus, the pka is easily determined from the titration curve just by noting the ph at the volume halfway to the equivalence point. In the algebraic method, sets of ph and absorbance values are substituted into eq 3 and the pka is calculated for each set. Combine the two to find the charge for the entire aa at a given ph. Then you can apply some equations to find the solution ph.
 Source: pinterest.com
Source: pinterest.com
An acid in a basic solution will ionize. If these values are known, then you can just put the values into this equation. An acid in a basic solution will ionize. It is not necessary to know the initial number of moles of ha in solution! We can use this same logic to find a pka value at any point** prior to the equivalence point.
 Source: pinterest.com
Source: pinterest.com
If ph > pka, then the species is deprotonated. Ph = pka + log 1/3 mol hai (after canceling mol hai) ph = pka + log 1 2/3 mol hai 2 note that only the ratio of moles is critical. H a ↽ − − ⇀ h x + + a x −. You will need to find a concentration first, however, on the equation. Combine the two to find the charge for the entire aa at a given ph.
 Source: pinterest.com
Source: pinterest.com
It is not necessary to know the initial number of moles of ha in solution! Ph calculation questions » finding pka of weak acid. At ph 8, the environment is considered acidic for phenol and it remains primarily. At a ph of 1, the environment is considered acidic and acetic acid exists predominately in its protonated form. It’s the negative logarithm of the proton concentration.
 Source: pinterest.com
Source: pinterest.com
It’s the negative logarithm of the proton concentration. If ph < pka, then the species is protonated. And solve for the dissociated hydrogen ion, say x. How to calculate ph from pka find ph from pka calculate pka from concentration pka ph formula ph = pka + log(base/acid) how to solve for pka how to go from ph to pka pka ph formula ph and pka equations using pka to find ph equation for pka ka ph equation determining ph from pka ph to ka calculator pka to ph conversion pka and ph equation ka to ph equation At ph 8, the environment is considered acidic for phenol and it remains primarily.
 Source: pinterest.com
Source: pinterest.com
It is not necessary to know the initial number of moles of ha in solution! If we look at the equation carefully, we need the pk a value then we need salt = acid, at that point the ph = pka so we just need to know what is the ph when the salt and acid concentration are equal. Ph —the measure of acidity. A small ka value means little of the acid dissociates, so you have a weak acid. Calculating ratio of conjugate base and acid when ph and pka are given.
 Source: pinterest.com
Source: pinterest.com
Because if y removes protons at a ph greater than 7, the ph of neutral h2o it is considered a base. If ph = pka, then half of the species has dissociated. It’s the negative logarithm of the proton concentration. If not, then there is no way to find the pka from the ph. Computing pk a from k a means performing the same operation as with ph:
 Source: pinterest.com
Source: pinterest.com
Then you can apply some equations to find the solution ph. Ph = pka + log 1/3 mol hai (after canceling mol hai) ph = pka + log 1 2/3 mol hai 2 note that only the ratio of moles is critical. If not, then there is no way to find the pka from the ph. A base in an acid solution will ionize. A small ka value means little of the acid dissociates, so you have a weak acid.
 Source: pinterest.com
Source: pinterest.com
Thus, the pka is easily determined from the titration curve just by noting the ph at the volume halfway to the equivalence point. If these values are known, then you can just put the values into this equation. This widget finds the ph of an acid from its pka value and concentration. A large ka value also means the formation of products in the reaction is favored. H a ↽ − − ⇀ h x + + a x −.
 Source: pinterest.com
Source: pinterest.com
A base in a basic solution will not ionize. H a ↽ − − ⇀ h x + + a x −. The pka is the ph at which the system consists of an equimolar concentration of the proton donor (ch 3 cooh) and proton acceptor (ch 3 coo¯). In the algebraic method, sets of ph and absorbance values are substituted into eq 3 and the pka is calculated for each set. You will need to find a concentration first, however, on the equation.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find pka from ph by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.